The US Food and Drug Administration (FDA) has greenlit the world’s inaugural vaccine for chikungunya, recognizing it as an “emerging global health threat.”
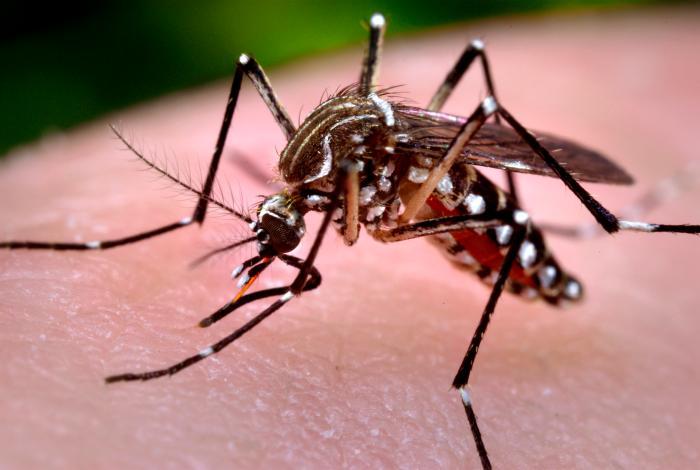
The disease, transmitted by mosquitoes, induces fever and joint pains and can be fatal for infants.
This approval is anticipated to accelerate the global distribution of the vaccine, known as Ixchiq, developed by Europe’s Valneva.
As of September this year, approximately 440,000 chikungunya cases, with 350 fatalities, have been reported.
The FDA sanctioned the vaccine for individuals aged 18 and older at high risk, and it involves a single-shot administration.
Chikungunya lacks a specific treatment, and regions such as South America and South Asia have observed elevated cases this year.
The disease’s symptoms also encompass rashes, headaches, and persistent joint pains for months or years.
Mosquitoes carrying the virus are endemic in tropical and subtropical regions of Africa, Southeast Asia, and parts of the Americas.
The FDA emphasized the disease’s increased prevalence in new geographic areas.
Brazil has reported the highest number of cases this year (218,613), with India noting over 93,000 cases, including a significant outbreak in Delhi in 2016.
Source-BBC